 |
Alternatives to Aging |
There are three main general alternative patterns to aging (age-related progressive fitness decline) (Finch 1990), which are not describable by Weibull’s equation:
First alternative: Clearly programmed death
Type 1: Rapid senescence and death after reproduction
Examples: Many species of salmons (Finch 1990, pp. 83-87), arachnids [ibidem, p. 76], mollusks [p. 78], lampreys [p. 80], some species of small marsupials [p. 95] and rodents [p. 97], many plants [p. 98], etc.
Type 2: Obligatory death for lack of anatomic parts in the adult stage
“Aphagy from defective mouthparts or digestive organs is very common during the adult phases of insects … and is the limiting factor in the adult lifespan of many short-lived species” [ibidem, p. 49], etc.
Type 3: Obligatory birth-related sudden maternal death
E.g.: “the nematode Ascaris nigrovenosa … the young kill their mother by boring through her body wall” [ibidem, p. 102], etc.
Second alternative: Negligible senescence (indefinite lifespan), negative senescence
Type 1: Negligible senescence
A species is defined as having negligible senescence when:
“(1) the age-dependent mortality rate remains relatively constant after maturity; or (2) there are negligible postmaturational functional impairments with advancing age.” (Finch 1990, p. 208)
Examples: “Certain conifers (gymnosperms) have enormous longevities of many thousand years in nature” [ibidem, p. 209]; “numerous arthropods show indeterminate growth and no signs of senescence” [p. 213]; many species of rockfish [http://www.agelessanimals.org/index.htm], “[There] are sponges and other asexually reproducing organisms in which most or all cells continue proliferation indefinitely and give the capacity for extensive regeneration of damaged organs; such species tend to have indefinite lifespans” [p. 61], etc.
Type 2: Negative senescence
In some cases a progressive increasing body mass, which reduces the possibility of being predated, or other factors reduce the mortality rate that otherwise would be presumably stable (Comfort 1979) (Congdon et al. 2003) (Vaupel et al. 2004). “Negative senescence”, a potentially misleading term, should be considered a particular case of negligible senescence.
Third alternative: Constant mortality in the wild and increasing mortality from ages not existing in the wild
The lack of cell turnover means that cells and organs cannot last forever by action of mechanical and biochemical factors progressively damaging organs or cells. E.g.: “insects [are] at risk for debilitating damage to irreplaceable cells and organs, particularly to their fragile wings and esoskeleton, which Comfort (1979) has called mechanical senescence” (Finch 1990).
If this lack of cell turnover causes organ / cell damage over a critical point from ages not existing in wild conditions, the lack of cell turnover is irrelevant for natural selection.
The fly Drosophila melanogaster and the nematode Caenorhabditis elegans, both composed of cells with no turnover (Finch 1990) (Arking 1998) are famous examples of this case.
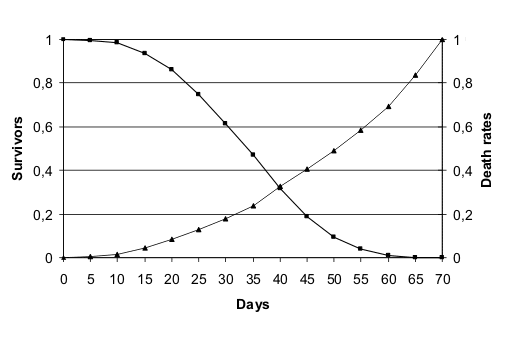
These two species show in laboratory conditions an age-related increasing mortality illustrate in fig. 1 (redrawn from (Finch 1990)) and fig. 2 (redrawn from (Finch & Hayflick 1977)).
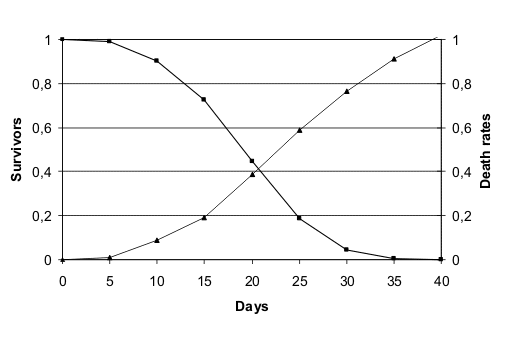
Life table in laboratory conditions of Caenorhabditis elegans.
Life table in laboratory conditions of Drosophila melanogaster.
However, the ages in which mortality increment starts in laboratory for C. elegans and D. melanogaster, do not exist in the wild. In fact, the longevity of C. elegans “under more natural conditions is reduced up to 10 fold compared with standard laboratory culture conditions” (Van Voorhies et al. 2005) and few individuals of this species remain fertile in the wild after 10 days (Johnson 1987). Similarly, D. melanogaster has a reported adult life span in the wild of 10-12 days (Finch 1990).
For both these animals, the age-related increasing mortality described in figures B1 and B2 starts at ages non-existent in the wild, meaning that it is only a laboratory artefact.
References:
- Arking R. (1998) Biology of aging. 2nd ed., Sinauer Associates, Sunderland, MA (USA). [Google Scholar]
- Comfort, A. (1979) The Biology of senescence. 3rd ed. Churchill Livingstone, Edinburgh and London. [Google Scholar]
- Congdon, J. D., Nagle, R. D., Kinney, O. M., van Loben Sels, R. C., Quinter, T., and Tinkle, D. W. (2003) Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp Gerontol 38(7):765-772. [PubMed] [Google Scholar]
- Finch, C.E. (1990) Longevity, Senescence, and the Genome. University of Chicago Press, Chicago. [Google Scholar]
- Finch C.E. and Hayflick L. (Eds.) (1977) Handbook of the biology of aging. Van Nostrand Reinhold Company, New York. [Google Scholar]
- Johnson T.E. (1987) Aging can be genetically dissected into component processes using long-lived lines of Caenorhabditis elegans. Proc. Natl. Acad. Sci. 84, 3777-81. [PubMed] [Google Scholar]
- Van Voorhies W.A., Fuchs J. and Thomas S. (2005) The longevity of Caenorhabditis elegans in soil, Biol. Letters 1, 247-9. [PubMed] [Google Scholar]
- Vaupel, J.W., Baudisch, A., Dolling, M., Roach, D.A. and Gampe, J. (2004) The case for negative senescence. Theor. Popul. Biol. 65(4):339-51. [PubMed] [Google Scholar]
www.programmed-aging.org
Sponsored by Azinet LLC © 2009