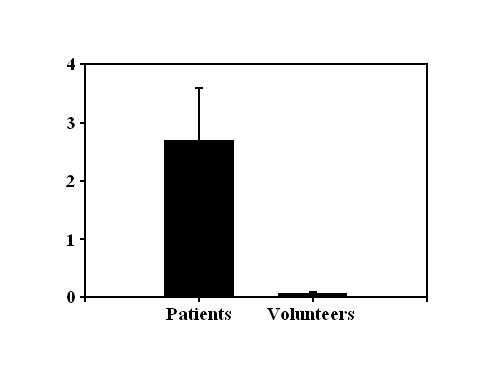
Quantitative PCR (qPCR) for Cyt B DNA
in plasma from trauma patients (μg/mL)
Mitochondrial debris including mitochondrial DNA
and formyl peptides that appear in the blood after major
trauma can induce a syndrome resembling sepsis
C.J. Hauser
MD, FACS, FCCM, Harvard Medical School, Boston
Injury causes a systemic inflammatory response syndrome ("SIRS") that is clinically much
like sepsis, and predisposes to organ failure, infection critical illness and death. SIRS is
responsible for more than half of intensive care morbidity and costs in the USA. It is known
that microbes present "pathogen-associated molecular patterns" (PAMPs) to the immune
system that activate innate immune cells through "pattern-recognition receptors" (PRR).
But it has recently been noted that cellular injury (especially non-apoptotic cell death)
can release endogenous "damage associated molecular patterns" (DAMPs) from cells
that also act on innate immunity. Part of this similarity is due to mitochondria being
evolutionary endosymbionts descended from bacteria.
We recently showed (Zhang et al., Nature, March 4, 2010) that mitochondria bear
bacterial molecular motifs including bacterial-style CpG-DNA and formylated peptides that
act upon human innate immune cells via Toll-Like Receptor-9 (TLR-9) and G-protein
coupled formyl peptide receptors. We have also showed that major injury releases
mitochondrial detritus into the circulation (Figure, left, shows qPCR for Cytochrome B
DNA) with mitochondrial DNA (mtDNA) reaching levels in the circulation that activate
immune cells.
Mitochondrial peptides and mtDNA signal through Formyl Peptide Receptor-1 and TLR9
respectively, promoting neutrophil (PMN) cytosolic calcium ([Ca2+]i) release and entry
as well as causing phosphorylation of P38- and P44/42-mitogen activated protein kinases
(MAPK).These signals cause PMN migration to and degranulation in the lung and liver,
initiating PMN-mediated oxidative organ injury there.
Thus cellular disruption by major trauma releases mitochondrial DAMPs into the circulation
that have evolutionarily conserved molecular signatures similar to bacterial PAMPs.
Release of such mitochondrial 'enemies within' locally is likely a necessary step in the
initiation of inflammation, which then leads to wound healing. In overwhelming injuries
however, the same pathways become activated systemically. In that case, widespread
activation of innate immunity leads to a SIRS syndrome that is similar to sepsis and has
a high lethality even with modern care. Thus innate immune activation is likely to be
beneficial at the local level in the case of minor, survivable injuries. But conversely,
global innate immune activation appears to promote early organ failure and death in the
case of major injuries that would not have been survivable early in the human evolutionary
process. Trauma surgeons who support these patients now with intensive care must
accept the SIRS response as a consequence of survival. But for early Man, it is likely
that in the case of major injuries selection pressures at the societal level would have
favored early death over prolonged illnesses that could have immobilized the entire
social group or exhausted its resources.

Quantitative PCR (qPCR) for Cyt B DNA
in plasma from trauma patients (μg/mL)
Homo Sapiens Liberatus Workshop, Moscow State University, May 2010